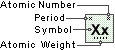Boron
Symbol
B
Atomic Number
5
Atomic Weight
10.811
Element Classification
Discovered By
Sir H. Davy, J.L. Gay-Lussac, L.J. Thenard
Discovery Date
1808 (England/France)
Name Origin
The Arabic and Persian words for borax.
Density (g/cc)
2.34
Melting Point (K)
2573
Boiling Point (K)
3931
Appearance
Hard, brittle, lustrous black semimetal
Atomic Radius (pm)
98
Atomic Volume (cc/mol)
4.6
Covalent Radius (pm)
82
Ionic Radius
23 (+3e)
Specific Heat
1.025
Fusion Heat (kJ/mol)
23.60
Evaporation Heat (kJ/mol)
504.5
Thermal Conductivity (@25°C W/m K)
27.4
Debye Temperature (K)
1250.00
Pauling Negativity Number
2.04
First Ionizing Energy (kJ/mol)
800.2
Oxidation States
3
Electronic Configuration
[He] 2s2 2p1
Lattice Structure
Tetragonal(TET)
Lattice Constant
8.730
Lattice C/A Ratio
0.576