Chemistry: Charles's Law: The Incredible Imploding Can
Charles's Law: The Incredible Imploding Can
Let's do another demonstration. You'll need a brand new, never used, metal can with a screw-on cap. Remove the cap, place the can on the stove, and turn it to "high." After the can has been heated for about two minutes, take it off the stove with metal salad tongs and tightly screw on the cap.
Because I know that none of you actually did the demonstration (shame on you!), I'll just tell you what you would have seen—over a period of two or three minutes, the can would shrink until the sides caved in.
Way back in 1787, the French scientist Jacques Charles did exactly the same experiment while sitting around the house on a rainy day. (Editor's note: Historians believe that only the year in the preceding statement is correct.) When he observed the can imploding, he devised the following law to explain his findings:
- V1⁄T1 = V2⁄T2
V1 is the initial volume of the can, T1 is the initial temperature of the air in Kelvin, V2 is the final volume of the can, and T2 is the final temperature of the air (in Kelvin). We will assume that the pressure and number of moles of the air are constant. If the can has an initial volume of 5.00 liters, the temperature of the air before you took the can off of the stove was 250º C (523 K), and the temperature of the air after the can cooled was 25º C (298 K), we can use this equation to find the final volume of the can:
Bad Reactions
When working with gases, remember to always convert temperatures from degrees Celsius to Kelvin (K = ºC + 273). If you don't, your answer will be wrong!
- 5L⁄523K = V2
- V2 = 2.85 L
What this equation means is that when the air inside the can cooled, the volume decreased, causing the can to implode. The kinetic molecular theory would explain this by saying that the air molecules had less kinetic energy at the lowered temperature, causing them to strike the sides of the can with less energy than they did before. Because the energy of the molecules hitting the sides of the can decreased, the pressure inside the can also decreased. When the pressure inside the can decreased, the much higher air pressure outside the can pushed in the sides of the can, causing it to implode.
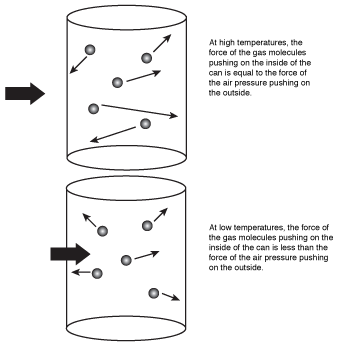
Figure 16.1The air molecules in the can hit the inside walls with less energy at low temperature, causing the can to implode as the air temperature decreases.
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







