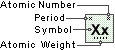Palladium
Symbol
Pd
Atomic Number
46
Atomic Weight
106.42
Element Classification
Discovered By
William Wollaston
Discovery Date
1803 (England)
Name Origin
Named after the asteroid, Pallas, discovered in 1803.
Density (g/cc)
12.02
Melting Point (K)
1825
Boiling Point (K)
3413
Appearance
Silvery-white, soft, malleable and ductile metal
Atomic Radius (pm)
137
Atomic Volume (cc/mol)
8.9
Covalent Radius (pm)
128
Ionic Radius
65 (+4e) 80 (+2e)
Specific Heat
0.244
Fusion Heat (kJ/mol)
17.24
Evaporation Heat (kJ/mol)
372.4
Thermal Conductivity (@25°C W/m K)
71.8
Debye Temperature (K)
275.00
Pauling Negativity Number
2.20
First Ionizing Energy (kJ/mol)
803.5
Oxidation States
4, 2, 0
Electronic Configuration
[Kr] 4d10
Lattice Structure
Face-Centered Cubic(FCC)
Lattice Constant
3.890
Lattice C/A Ratio
n/a