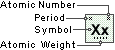Silicon
Symbol
Si
Atomic Number
14
Atomic Weight
28.0855
Element Classification
Discovered By
Jons Jacob Berzelius
Discovery Date
1824 (Sweden)
Name Origin
Latin: silex, silicus, (flint).
Density (g/cc)
2.33
Melting Point (K)
1683
Boiling Point (K)
2628
Appearance
Amorphous form is brown powder; crystalline form has a gray
Atomic Radius (pm)
132
Atomic Volume (cc/mol)
12.1
Covalent Radius (pm)
111
Ionic Radius
42 (+4e) 271 (-4e)
Specific Heat
0.703
Fusion Heat (kJ/mol)
50.6
Evaporation Heat (kJ/mol)
383
Thermal Conductivity (@25°C W/m K)
149
Debye Temperature (K)
625.00
Pauling Negativity Number
1.90
First Ionizing Energy (kJ/mol)
786.0
Oxidation States
4, -4
Electronic Configuration
[Ne] 3s2 3p2
Lattice Structure
Diagonal(DIA)
Lattice Constant
5.430
Lattice C/A Ratio
n/a