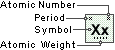Bromine
Symbol
Br
Atomic Number
35
Atomic Weight
79.904
Element Classification
Discovered By
Antoine J. Balard
Discovery Date
1826 (France)
Name Origin
Greek: bromos (stench).
Density (g/cc)
3.12
Melting Point (K)
265.9
Boiling Point (K)
331.9
Appearance
Reddish-brown liquid
Atomic Radius (pm)
n/a
Atomic Volume (cc/mol)
23.5
Covalent Radius (pm)
114
Ionic Radius
47 (+5e) 196 (-1e)
Specific Heat
0.473 (Br-Br)
Fusion Heat (kJ/mol)
10.57 (Br-Br)
Evaporation Heat (kJ/mol)
29.56 (Br-Br)
Thermal Conductivity (@25°C W/m K)
0.005
Debye Temperature (K)
n/a
Pauling Negativity Number
2.96
First Ionizing Energy (kJ/mol)
1142.0
Oxidation States
7, 5, 3, 1, -1
Electronic Configuration
[Ar] 3d10 4s2 4p5
Lattice Structure
Orthorhombic(ORC)
Lattice Constant
6.670
Lattice C/A Ratio
n/a