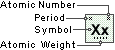Magnesium
Symbol
Mg
Atomic Number
12
Atomic Weight
24.305
Element Classification
Discovered By
Sir Humphrey Davy
Discovery Date
1808 (England)
Name Origin
Magnesia, ancient city in district of Thessaly, Greece.
Density (g/cc)
1.738
Melting Point (K)
922
Boiling Point (K)
1363
Appearance
Lightweight, malleable, silvery-white metal
Atomic Radius (pm)
160
Atomic Volume (cc/mol)
14.0
Covalent Radius (pm)
136
Ionic Radius
66 (+2e)
Specific Heat
1.025
Fusion Heat (kJ/mol)
9.20
Evaporation Heat (kJ/mol)
131.8
Thermal Conductivity (@25°C W/m K)
156
Debye Temperature (K)
318.00
Pauling Negativity Number
1.31
First Ionizing Energy (kJ/mol)
737.3
Oxidation States
2
Electronic Configuration
[Ne] 3s2
Lattice Structure
Hexagonal(HEX)
Lattice Constant
3.210
Lattice C/A Ratio
1.624