Chemistry: Properties of Ionic Compounds
Properties of Ionic Compounds
Because all ionic compounds are formed when anions and cations are attracted to one another, ionic compounds frequently have similar characteristics.
Ionic Compounds Form Crystals
Ionic compounds consist of cations and anions that stick next to each other because of their opposite charges. Imagine a single lithium cation stuck next to a single chlorine anion to form lithium chloride. Now, it's unlikely that only one lithium ion and one chloride ion will be present in this location—generally, when we speak of chemical reactions, we're talking about a huge number of atoms undergoing a reaction in a very small place (one teaspoon of salt contains approximately 1022 atoms). As a result, if our single LiCl pair were to come close to another LiCl pair, the following would take place:
Because oppositely charged ions attract one another, the LiCl pairs will tend to form larger groups. These larger groups, in turn, will form even larger groups of ions, as shown in the following figure:
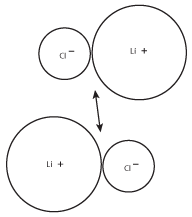
Figure 8.3The positive charge on the lithium cation of one pair will be attracted to the negative charge on the chloride ion of the other pair.
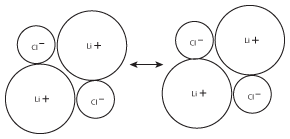
Figure 8.4This process, where stacks of LiCl ions combine with one another, will continue until there are no more lithium or chloride ions.
Molecular Meanings
Crystals are large arrangements of ions or atoms that are stacked in regular patterns. Many ionic compounds form very large crystals.
These large arrangements of ions are referred to as crystals. Though crystals are frequently formed from ionic compounds, they also exist in some other chemical compounds, such as diamonds. We'll talk about this in much greater detail in Solids.
Chemistrivia
Hydrates are formed when one or more molecules of water attach themselves to ionic compounds. These compounds are interesting because they appear dry but give off water when heated. Particularly interesting is Epsom salt, or magnesium sulfate heptahydrate (MgSO4·7H2O). When heated, enough water is given off that it actually dissolves the magnesium sulfate!
Ionic Compounds Often Have High Melting and Boiling Points
What happens when you heat something up in your kitchen? You may have discovered while cooking (or while microwaving random things while bored) that most of the foods we eat either melt or burn when heated. Some foods even do both! As you can probably guess, I'm an expert when it comes to putting out house fires.
Ionic compounds, on the other hand, frequently melt and boil at much higher temperatures than other materials. In order for ionic compounds to melt, enough energy must be added to make the cations and anions move away from one another. Because these attractions are so strong, it takes a lot of energy to pull these ions apart. Adding this much energy to ionic compounds requires a great deal of heat, which is why ionic compounds have very high melting and boiling points.
Ionic Compounds Are Hard and Brittle
Imagine bashing a big chunk of lithium chloride against your head. What do you suppose that might feel like? If you guessed that it would hurt like crazy, you were right. Like many ionic compounds, lithium chloride is as hard as a rock.
Ionic compounds are extremely hard because it is difficult to make the ions move apart from each other in a crystal. Even if you apply a great deal of force on the crystal (imagine running headlong into a giant wall of lithium chloride), the attraction between the cations and anions will frequently continue to hold the crystal together.
Let's say, though, that you really want to break apart an ionic compound. While very hard, ionic compounds are also frequently very brittle, meaning that they break apart when the right kind of force is applied. As the following figure shows, where you apply the force is just as important as how much force you use.
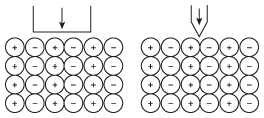
Figure 8.5By applying force in a way that pries the cations and anions apart from each other: you can cause a crystal to completely break apart.
As you can see from this diagram, ionic crystals align themselves such that there are regions where a small force can break apart the crystal. These regions are sometimes referred to as "cleavage planes" because they are the locations where the crystal is weakest and can most easily be broken.
Ionic Compounds Conduct Electricity When Dissolved in Water or Melted
Once upon a time, there was an inventor who came up with a device for drying hair. This "hairdryer" as he called it, heated air with electricity and blew it across the hair of the person holding it. Because water evaporates when heated, the hair dried more quickly. This inventor's legacy lives on to this day in a household appliance loved by millions.
Molecular Meanings
Electrolytes are compounds that conduct electricity when dissolved in water. Many ionic compounds are considered to be electrolytes. However, some ionic compounds don't dissolve in water. As a result, they don't share this property.
Shortly afterward, there was a guy who decided that he didn't want to wait to get out of the bathtub before drying his hair. His legacy: A hairdryer warning sticker with a picture of a guy getting electrocuted.
When ionic compounds are placed in water, they cause the water to conduct electricity. Normally, water doesn't conduct electricity well at all. However, when salts dissolve in water, they break up into their constituent cations and anions and it is the presence of these ions that allows it to conduct electricity. Because salts conduct electricity when dissolved in water, they are referred to as electrolytes.
In the same way, pure salts also conduct electricity when they are melted. As a solid, the anions and cations in an ionic compound are locked in place and unable to move electrical charge. However, when the ionic compound is melted, these ions are free to move around and conduct charge.
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







