Chemistry: How and Why Do Things Dissolve?
How and Why Do Things Dissolve?
When making a solution, it's handy to know if one thing will dissolve in another. After all, if somebody wants you to make them a liquid solution of one chemical, it won't make you look good if you bring them a beaker of liquid with sludge sitting at the bottom because you chose the wrong solvent.
The best way to tell if something will dissolve is to look at the polarities of the solvent and the solute. If the polarities of the solvent and solute match (both are polar or both are nonpolar), then the solute will probably dissolve. If the polarities of the solvent and solute are different (one is polar, one is nonpolar), the solute probably won't dissolve. Let's explore why this happens.
The Mole Says
The phenomenon that polar solvents dissolve ionic and polar solutes, nonpolar solvents dissolve nonpolar solutes, and polar solvents don't dissolve nonpolar solutes (and vice-versa) is often summed up by the phrase "Like dissolves like."
Why Polar Solvents Dissolve Ionic and Polar Solutes
As mentioned earlier, polar solvents are good at dissolving polar solutes. To explain this, we'll describe the process that occurs when table salt (sodium chloride) dissolves in water.
As we learned in The Mole, water is a polar molecule with partial positive charge on each hydrogen atom and partial negative charge on the oxygen atom. This polarity is shown in the following figure:
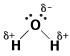
Figure 14.1Water is a polar covalent molecule that's good at dissolving polar solids.
Chemistrivia
In some cases, the attraction of water molecules for the polar solute isn't strong enough to pull the solute molecules apart. As a result, some polar solutes don't dissolve in water.
Ionic solids like sodium chloride, by definition, contain cations and anions. As a result, when an ionic solid such as sodium chloride is placed into water, we see the following take place:
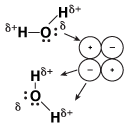
Figure 14.2Sodium chloride is highly soluble in water.
When sodium chloride is placed into water, the partial positive charges on the hydrogen atoms in water are attracted to the negatively charged chloride ions. Likewise, the partial negative charges on the oxygen atoms in water are attracted to the positively charged sodium ions. Because the attractions of the water molecules for the sodium and chloride ions are greater than the forces holding the crystal together, the salt dissolves. When a solute dissolves in water, the process is referred to as hydration.
Similarly, we find that polar solutes such as methanol, ethanol, and isopropanol are highly soluble in water because both are highly polar.
Why Polar Solvents Don't Dissolve Nonpolar Solutes
The "like dissolves like" rule indicates that polar solvents will do a poor job of dissolving nonpolar solutes. We can understand this by looking at the following figure:
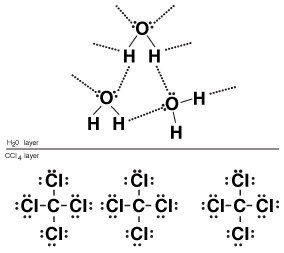
Figure 14.3Water doesn't dissolve carbon tetrachloride because the strong interactions between water molecules are more important than the weak interactions between water and carbon tetrachloride.
In the preceding figure, we can see what happens when we place carbon tetrachloride into water. Because carbon tetrachloride is a nonpolar molecule, the interactions between adjacent molecules are very weak. As a result, we might expect carbon tetrachloride to be very soluble in water. However, water molecules form strong hydrogen bonds with one another, causing them to stick tightly to one another. Since the water molecules have very strong intermolecular forces with each other and interact only weakly with carbon tetrachloride (via London dispersion forces—see Liquids and Intermolecular Forces), CCl4 is almost completely insoluble in water.
Why Nonpolar Solvents Don't Dissolve Polar Solutes
Let's imagine what happens when a polar solute such as sodium chloride is placed in a nonpolar solvent such as carbon tetrachloride. Because CCl4 doesn't have a partial charge, it won't attach itself to the sodium or chloride ions. As we've mentioned before, the sodium and chloride ions in NaCl are strongly attracted to one another because of their opposite charges. This very weak solvent-solute interaction, as well as the very strong attraction between neighboring solute particles, causes sodium chloride to be insoluble in carbon tetrachloride.
Why Nonpolar Solvents Dissolve Nonpolar Solutes
If we place a nonpolar solid into a nonpolar liquid, "like dissolves like" implies that the solid will dissolve. However, the only forces that will cause the liquid to be attracted to the solid are weak London dispersion forces. Why should the solid dissolve?
Let's imagine that we have placed a chunk of carbon tetrabromide in a beaker containing carbon tetrachloride. The carbon tetrabromide molecules in the solid are held together by very weak London dispersion forces, as are the carbon tetrachloride molecules in the solvent. One might expect, then, that there is no particular reason for the solute to dissolve.
As it turns out, there's another force involved. Processes that increase the randomness of a system usually occur spontaneously (we'll discuss this phenomenon, known as entropy. Because the molecules in carbon tetrabromide will be made more random if they're mixed with another compound, the carbon tetrabromide will dissolve in the carbon tetrachloride.
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







