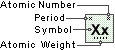Hydrogen
Symbol
H
Atomic Number
1
Atomic Weight
1.00794
Element Classification
Discovered By
Henry Cavendish
Discovery Date
1766 (England)
Name Origin
Greek: hydro (water) and genes (generate)
Density (g/cc)
0.0708 (@ -253�C)
Melting Point (K)
14.01
Boiling Point (K)
20.28
Appearance
Colorless, odorless, tasteless gas
Atomic Radius (pm)
79
Atomic Volume (cc/mol)
14.1
Covalent Radius (pm)
32
Ionic Radius
154 (-1e)
Specific Heat
14.267 (H-H)
Fusion Heat (kJ/mol)
0.117 (H-H)
Evaporation Heat (kJ/mol)
0.904 (H-H)
Thermal Conductivity (@25°C W/m K)
0.1815
Debye Temperature (K)
110.00
Pauling Negativity Number
2.20
First Ionizing Energy (kJ/mol)
1311.3
Oxidation States
1, -1
Electronic Configuration
1s1
Lattice Structure
Hexagonal(HEX)
Lattice Constant
3.750
Lattice C/A Ratio
1.731