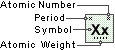Sodium
Symbol
Na
Atomic Number
11
Atomic Weight
22.989768
Element Classification
Discovered By
Sir Humphrey Davy
Discovery Date
1807 (England)
Name Origin
Medieval Latin: sodanum, (headache remedy), symbol from Latin natrium, (sodium carbonate).
Density (g/cc)
0.971
Melting Point (K)
370.96
Boiling Point (K)
1156.1
Appearance
Soft, silvery-white metal
Atomic Radius (pm)
190
Atomic Volume (cc/mol)
23.7
Covalent Radius (pm)
154
Ionic Radius
97 (+1e)
Specific Heat
1.222
Fusion Heat (kJ/mol)
2.64
Evaporation Heat (kJ/mol)
97.9
Thermal Conductivity (@25°C W/m K)
142.0
Debye Temperature (K)
150.00
Pauling Negativity Number
0.93
First Ionizing Energy (kJ/mol)
495.6
Oxidation States
1
Electronic Configuration
[Ne] 3s1
Lattice Structure
Body-Centered Cubic(BCC)
Lattice Constant
4.230
Lattice C/A Ratio
n/a