Chemistry: What's in a Name? Ionic Nomenclature
What's in a Name? Ionic Nomenclature
If you've been doing chemistry for any length of time, you're well aware of the difficulty posed with naming ionic compounds. Anybody who has needed CuCl2 and found that the only chemicals available are "cuprous chloride" and "cupric chloride" knows how much trouble naming ionic compounds can be. Fortunately, we're going to discuss a method for making ionic naming simple and easy.
Before we get started with naming, we need to learn about polyatomic ions. As the name suggests, polyatomic ions are ions that contain more than one atom. An example of a polyatomic ion is the hydroxide ion, which has the formula OH-1; together, the oxygen and hydrogen have a net -1 charge. Generally, polyatomic ions are anions, though the ammonium and mercury(I) cations are exceptions. Polyatomic ions are found in many ionic compounds, making it important that you learn the names and formulas of the more common ions:
| Name of Ion | Formula and Charge of Ion |
| acetate | C2H3O2-1 |
| ammonium | NH4+1 |
| bicarbonate | HCO3-1 |
| bisulfate | HSO4-1 |
| carbonate | CO3-2 |
| chlorate | ClO3-1 |
| chlorite | ClO2-1 |
| chromate | CrO4-2 |
| cyanide | CN-1 |
| dichromate | Cr2O7-1 |
| hydroxide | OH-1 |
| mercury(I) | Hg22+ |
| nitrate | NO3-1 |
| nitrite | NO2-1 |
| permanganate | MnO4-1 |
| phosphate | PO4-3 |
| sulfate | SO4-2 |
| sulfite | SO3-2 |
Writing Ionic Names from Formulas
Now that we're familiar with polyatomic ions, let's learn how to name ionic compounds when given their chemical formulas by using the following steps:
Step 1
Determine the "base name" of the ionic compound. Ionic compound base names contain two words:
- The first word is the name of the cation. Unless the cation is "ammonium" (in which case you already know its name), the name of the cation is the same as the name of the element. For example, the first word in "NaOH" is "sodium."
- The second word is the name of the anion. If the anion is a polyatomic ion, you can just look up the name on the chart of polyatomic ions shown earlier. For example, "NaOH" is "sodium hydroxide." If the anion is a single element, replace the ending of the element name with "-ide." For example, NaBr would be "sodium bromide."
Step 2
Determine whether or not the compound will require a Roman numeral.
For many compounds, you can stop with the base name. However, some elements that form cations can have more than one possible charge. For example, iron can form two ionic compounds with chlorine; "FeCl2" and "FeCl3." Because the naming system we just learned would call both of these compounds "iron chloride," we need some way to distinguish between them. To do this, we write a Roman numeral after the name of the cation to indicate the amount of positive charge it has.
Bad Reactions
Use Roman numerals only when naming ionic compounds that have cations with more than one possible positive charge. If you place Roman numerals in all compound names, they will be wrong when misapplied.
Unfortunately, we can't just go putting Roman numerals for all ionic compounds. We can only do it for compounds containing cations that commonly bear more than one possible charge. To help us with this job, take a look at the following figure, which shows the positive charges of many common cations.
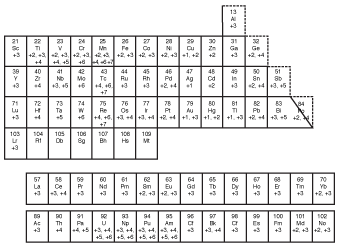
Figure 8.6Use this diagram to determine which elements have only a single possible charge and which elements can exist with more than one possible positive charge.
As you can see, some elements can have several different possible positive charges. Cobalt (Co), for example, can have either a charge of +2 or +3, making it necessary to use Roman numerals to distinguish between them. Zinc, on the other hand, doesn't require Roman numerals in its compound names because it only forms stable cations with a charge of +2.
Step 3
Determine the Roman numeral that goes after the cation name.
To do this, use the following formula:
- Roman numeral = -(charge on anion) × (number of anions)⁄(number of cations)
You've Got Problems
Problem 2: Name the following ionic compounds using the previous rules:
(a) Na2SO4
(b) Cu2O
(c) CoCO3
(d) NH4Cl
Let's see how that works with the examples FeCl2 and FeCl3, both of which have the base name "iron chloride."
FeCl2 contains two chloride ions, each of which has a negative charge of 1. Because there is no subscript under the Fe, there is only one iron atom present. As a result, the Roman numeral required for FeCl2 is: -(-1)(2)⁄1 = 2, making this compound iron(II) chloride.
FeCl3 contains three chloride ions with negative charges of 1. Because there is only one iron atom, the Roman numeral for FeCl3 is: -(-1)(3)⁄1 = 3
The name of FeCl3 is iron(III) chloride.
Writing Ionic Formulas from Names
As you might have guessed, writing formulas from names is pretty much the reverse process of writing names from formulas. Let's learn the steps:
Step 1
From the base name, determine the formula and charge of the ions.
Let's say we were told to write the formula for "calcium sulfate." From the name "calcium" we know that the cation will be Ca+2. The "Ca" part is simply the atomic symbol for calcium, and the "+2" is given to us by the octet rule because calcium needs to lose two electrons to get the same electron configuration as argon.
The Mole Says
If you have more than one polyatomic ion, you must always put parentheses around it before placing the subscript under it. "Beryllium hydroxide" is Be(OH)2, not BeOH2. However, if the ion is not polyatomic, never use parentheses!
Because sulfate isn't an element on the periodic table, many people start screaming in panic and confusion. If you get into a situation where an unfamiliar ion shows up, take a look at the chart of polyatomic ions and see if the ion is listed there. As it turns out, sulfate is the SO4-2 ion. Feel better?
You've Got Problems
Problem 3: Write the formulas of the following ionic compounds:
(a) lithium acetate
(b) sodium nitrate
(c) chromium(VI) sulfate
(d) zinc phosphate
Step 2
Write the formulas of the cations and the anions next to each other.
This isn't so bad! In our example, we just write Ca+2 SO4-2.
Step 3
Devise an ionic formula that gives the compound a neutral charge.
In our example, the charges on the calcium cation and sulfate anion cancel each other. As a result, the compound will be electrically neutral when one calcium ion combines with one sulfate ion, forming CaSO4.
Let's go through another example: beryllium hydroxide.
- "Beryllium" indicates Be+2 and "hydroxide" indicates OH-1.
- Putting them together, we get Be+2 OH-1.
- Because beryllium hydroxide has to be electrically neutral, there need to be two hydroxide ions for each beryllium ion. As a result, the formula of beryllium hydroxide is Be(OH)2.
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







