Chemistry: Important Terms and Units
Important Terms and Units
Because we've just started dealing with gases, it's time to learn some new terms for working with them. These terms will be unbelievably handy, so make sure that you understand them before moving on!
Pressure
Pressure is defined as being the amount of force exerted by the particles in a gas as they hit the sides of a container. Let's imagine that we have five gas particles in a box, as shown in the following figure:
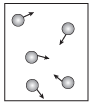
Figure 15.3Gas pressure is caused by the force of gas molecules crashing into the sides of the container that holds them.
As the kinetic molecular theory described, these particles go whizzing around at unbelievably high speeds in random directions. Whenever the particles hit the walls, the force of the impact results in gas pressure. For those of you who want to experience this phenomenon at home, have a friend throw tennis balls at you for a few minutes. The force that drives you backward is the pressure of the tennis balls.
There are several different units of pressure that are commonly used.
Chemistrivia
The unit "torr" was named after Evangelista Torricelli, an Italian physicist who worked with gases. Among other things, Torricelli is credited with inventing the barometer.
- Atmospheres (atm): 1 atm is defined as the average atmospheric pressure at sea level. Though it's not an official metric unit, it's frequently used because it's so darn handy.
- Millimeters of mercury (mm Hg) or torr: Both of these units are identical. There are 760 mm Hg or torr in 1 atm.
- Pascals (Pa): This is the metric unit of pressure. There are 1.01325 × 105 Pa in 1 atm. Typically, we use the unit "kilopascals" (kPa) instead of pascals. There are 101.325 kPa in 1 atm.
- Bar: The bar is a unit of pressure most commonly used by meteorologists. There are 1.01325 bars in 1 atm.
- Pounds per square inch (psi): Though not a metric unit, it's still in widespread use for many purposes including automobile tire pressures. Unlike other units, psi indicates how much gas pressure is present in excess of one atm. There are approximately 14.7 psi in 1 atm.
Volume and Temperature
When working with gases, volume is expressed in "liters" (L). Temperature is expressed in Kelvin (K).
Other Miscellaneous Terms
In addition to the preceding terms, there are several other terms that will come in handy when working with gases:
- Standard Temperature and Pressure (STP): STP is the most common reference condition for expressing the properties of gases. Standard temperature is defined as 0º C (273 K), and standard pressure is 1 atm.
- The ideal gas constant (a.k.a. "universal gas constant"): Shown as "R" in equations. It has two values that are useful for our calculations: 0.08206 L atm/mol K, and 8.314 L kPa/mol K. The value you should use for a particular problem depends on the units of pressure (kPa or atm) you're given.
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







