DK Science: Chemical Reactions
In a chemical reaction, the molecules of one substance break apart and join together with those of another substance to create a different compound (combination of molecules). Many chemical reactions are NON-REVERSIBLE CHANGES .You cannot turn a baked cake back into its raw ingredients. Some chemical reactions can be reversed, and re-formed into the original substances. These are REVERSIBLE CHANGES.
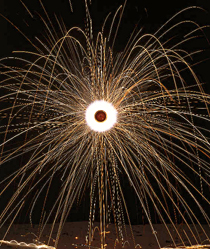
When the iron and magnesium in a firework burn, they react with oxygen and produce ash and smoke. They also release spectacular heat, light, and noise. Chemical changes produce new materials. They also usually give out or take in energy such as heat or light because chemical bonds have been broken and made.

When iron rusts, it reacts with oxygen in water or in air to create a new compound called iron oxide (rust). As in every chemical reaction, no mass is lost or gained. The same atoms from the original material are in the new materials, but in different places. If you weighed the iron oxide in this rusting ship, it would weigh the same as the original iron and oxygen.
A melting ice lolly is an example of a physical change, not a chemical change. The liquid ice lolly is not a new material, just a different form of the old one. Physical changes do not create new substances and no chemical bonds are broken or made. Melting, freezing, tearing, bending, and crushing are all physical changes that alter a substance’s appearance but not its chemical properties.
Many chemical reactions are non-reversible changes. This means they are permanent changes that cannot be undone. You cannot turn the new materials made back into the original materials again. Rusting is a non-reversible change. However, if rust is mixed with magnesium powder another chemical reaction occurs and iron can be extracted from the rust.

Decomposing (rotting) of food is a non-reversible reaction. Tiny living things called micro-organisms feed on the food and turn it into other substances, including nitrogen compounds and carbon dioxide. It is impossible to re-create fresh food from rotten food. This process is called decomposition because complex compounds are splitting up into simpler compounds.
A few chemical reactions can be reversed – the original materials can be re-created from the new materials. These reactions are called reversible changes. They have a forward reaction and a backward reaction. Both reactions are actually happening at the same time but, depending on the conditions, one will be stronger than the other.
When the gas nitrogen dioxide is heated, a forward chemical reaction changes the brown nitrogen dioxide gas into two colourless gases – nitrogen monoxide and oxygen. However, if these colourless gases are cooled, they will re-form into brown nitrogen dioxide gas. This is called a backward chemical reaction.
