Chemistry: What Are Covalent Compounds?
What Are Covalent Compounds?
In Ionic Compounds, we learned that ionic compounds are formed when an electronegative atom grabs an electron from an atom with low electronegativity. The reason for this is the octet rule, which states that all elements want to gain or lose electrons so they have the same electron configuration as the closest noble gas.
One thing we didn't discuss, however, is what happens when two electronegative atoms react with one another. For example, both nitrogen and hydrogen want to gain electrons to be like their nearest noble gas—this suggests that they won't give electrons to one another. By doing so, one would actually be further away from its goal of a full electron shell. Surprisingly, hydrogen and nitrogen actually form a large number of chemical compounds with one another, including everybody's favorite household cleaner, ammonia (NH3). How does this work, anyway?
I'm glad you asked! Let's imagine the following scenario:
Iodine wants another electron to be like its nearest noble gas, xenon. Hydrogen also wants another electron to be like its nearest noble gas, helium. What happens when a neutral hydrogen atom bumps into a neutral iodine atom? They do exactly what we were all taught to do in kindergarten—they share!
Molecular Meanings
Valence electrons are the number of s- and p-electrons since the most recent noble gas. These electrons are primarily responsible for the reactivities of the elements in the s- and p-sections of the periodic table. Covalent bonds are the bonds created when two valence electrons are shared.
Let's take a quick break from this example to define a couple of terms that will enable us to understand how this is relevant in the real world of atoms and electrons:
- Valence electrons are the number of s- and p-electrons past the most recent noble gas. For example, lithium has one valence electron and nitrogen has five valence electrons. These valence electrons are important because all elements tend to want to have the same number of valence electrons as the nearest noble gas (our buddy the octet rule), and these valence electrons determine the reactivities of many chemical compounds. Elements generally want a total of eight valence electrons to fill their outermost s- and p-orbitals; important exceptions are hydrogen, beryllium, and lithium, each of which wants two valence electrons to be like helium.
- Covalent bonds are the bonds formed when two atoms share a pair of valence electrons. All covalent bonds contain two electrons.
Back to our example:
Iodine has seven valence electrons, making it one short of the eight valence electrons it needs in order to be like xenon. Hydrogen has one valence electron, making it one short of the two valence electrons it needs in order to be like helium.
Chemistrivia
Oxygen contains two pairs of electrons that don't bond at all. These electron pairs are referred to as "unshared electron pairs," "lone pairs," or "unbonded pairs."
Here's the neat part: If hydrogen and iodine share their valence electrons, they can both pretend that they have the same number of valence electrons needed to be like their nearest noble gases. Here's a drawing of what this looks like:

Figure 9.1Before iodine and hydrogen combine, they're both missing the one valence electron they need to have the same electron configuration as the nearest noble gas. By sharing their unpaired valence electron, each pretends it has the right number of valence electrons—by doing so, they form a covalent bond.
The Mole Says
You may have noticed that this figure initially shows the electrons on oxygen spread out on all four sides rather than fully paired in three positions. We show electrons unpaired whenever possible because Hund's rule states that electrons prefer to remain unpaired (The Modern Atom).
From the preceding figure, we can see that both atoms simulate having the right number of valence electrons by sharing their unpaired electron with the other. The left side of the figure shows that iodine has only seven valence electrons. However, once it bonds with hydrogen, it has eight valence electrons around it. Of course, the number of electrons doesn't change, but any shared electrons count toward both the valence electrons for both atoms. Likewise, hydrogen has two valence electrons around it, making it stable.
Let's see what happens when hydrogen combines with oxygen to form water, H2O.
Each hydrogen atom has only one valence electron. In order to get the desired two valence electrons, each needs to gain another electron. Oxygen, however, has only six valence electrons. To be like neon with its eight valence electrons, it needs to gain an additional two. The following figure illustrates how one oxygen atom and two hydrogen atoms bond to form H2O:
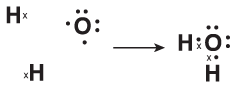
Figure 9.2
When two hydrogen atoms share one electron each with an oxygen atom, the three atoms form a chemical compound with two covalent bonds.As you can see, oxygen has two unpaired electrons that need to be paired up in order to be like neon. As in our previous example, each hydrogen atom needs one more electron to be like helium. The problem is solved when both hydrogen atoms form covalent bonds with oxygen, forming H2O.
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







