Anatomy and Physiology: Gas Laws and Breathing
Gas Laws and Breathing
Every chemistry student learns three basic gas laws: Charles's law, Boyle's law, and Dalton's law. In terms of respiration, Charles's law is the least applicable since body temperature rarely changes by much. Charles's law states the given constant pressure as the temperature of the gas increases so does the pressure. Boyle's and Dalton's laws, however, very much apply.
Contents Under Pressure
Boyle's law refers to a simple inverse relationship between volume and pressure. As thevolume of a container increases, the pressure of the gas within the container decreases.Conversely, a decrease in the size of a container will increase the pressure of the gas inside. In terms of containers, think about the thoracic cavity. The thoracic cavity is enclosed by the rib cage and by the diaphragm. Although the inside of the lungs is directly open to the outside environment, the area outside the lungs is not. As you will see, this is very important in your ability to breathe.
Flex Your Muscles
A great illustration of this is to take an empty 2-liter soda bottle with the cap off and squeeze it. It doesn't put up much resistance. Now put the cap on and try to squeezeit again. At this point, with the enclosed container, the gas molecules inside areaffected by the change in volume, increase the pressure, and push back against themuscles of your hand.
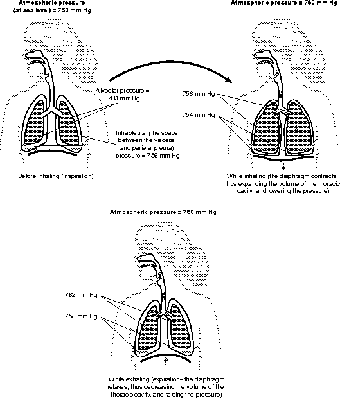
Figure 13.7The control of gas pressure (through Boyle's law) is controlled by the contraction of the diaphragm and the rib cage.
Don't Stop Bellowing
Many muscles are involved in breathing. Some, such as the internal and external intercostal muscles, control the movement of the rib cage; others control the movement of the abdomen. The most important muscle, however, in breathing is the diaphragm. The diaphragm is a dome-shaped muscle, and like every muscle, it moves by contracting. The dome shape is important because when the diaphragm contracts, the curve of the dome becomes flatter and shallow. As such, when the diaphragm contracts, the thoracic cavity gets bigger (see Figure 13.7).
The increase in the volume of the thoracic cavity means a slight drop in pressure. At this point the pressure of the outside air is slightly higher than the pressure inside the thoracic cavity. Since pressure moves, just like diffusion, from high to low, the outside air rushes in and fills the lungs. The dome shape kicks in again when the diaphragm relaxes, because relaxation causes the diaphragm to resume its dome shape in a process known as elastic rebound. This reduces the volume of the thoracic cavity, increases its pressure, and forces the air out. The change in pressure need not be large, however, for in normal breathing the pressure inside the thoracic cavity changes only slightly: air pressure at sea level = 760 mm Hg (mercury), normal inhalation = 759 mm Hg, normal exhalation = 761 mm Hg!
Crash Cart
One of the most common mistakes students make is to refer to “sucking in air,” or “sucking on a straw.” When people think of suction, they think that the air is being pulled into the straw, or into the vacuum cleaner. Nothing could be further from the truth! The lower pressure inside the mouth allows the higher pressure in the air to push down on the surface of the fluid you are drinking, thus pushing the fluid up the straw. There is no “sucking force” in science, also known as “Nothing Sucks in Science!”
Dalton's Law and Partial Pressure
Dalton's law states that each of the gases in a gas solution (such as air) exerts its own pressure based upon its concentration in the solution (see Figure 13.8). The air you breathe is made predominantly of two gases: nitrogen (78.6 percent) and oxygen (20.9 percent). The rest, only 0.5 percent, is mostly water, although in the summer on the East Coast it sure feels like more! Surprisingly, carbon dioxide is only 0.04 percent of the air!
Using “P” or “p” for partial pressure, air follows this formula for partial pressure:
- pN2 + pO2 + pH2O + pCO2 = 760 mm Hg
with the percentages from above:
- 597 mm + 159 mm + 3.7 mm + 0.3 mm = 760 mm Hg
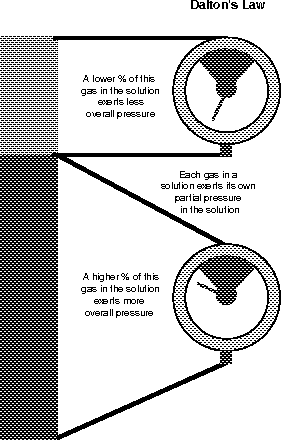
Figure 13.8Each of the different gases in the air exerts a different partial pressure (Dalton's law). (©Michael J. Vieira Lazaroff)
In the alveoli, the carbon dioxide reaches up to 5.2 percent. With a partial pressure of 40 mm Hg, this is 1,000 times higher than the pressure in the air! As you can imagine, you have no problem getting rid of carbon dioxide! On the other hand, the levels of oxygen drop as low as 13.2 percent, or a partial pressure of 100 mm Hg. Clearly you don't use all the oxygen with every breath—good news for people trapped with a limited supply of oxygen!

Excerpted from The Complete Idiot's Guide to Anatomy and Physiology © 2004 by Michael J. Vieira Lazaroff. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







